Cutimed® DebriClean
is a mechanical debridement pad which consists of two sorts of looped fibers, soft monofilament fibers for gentle abrasion that protect intact tissue and more abrasive fibers to break up and remove even firm slough.
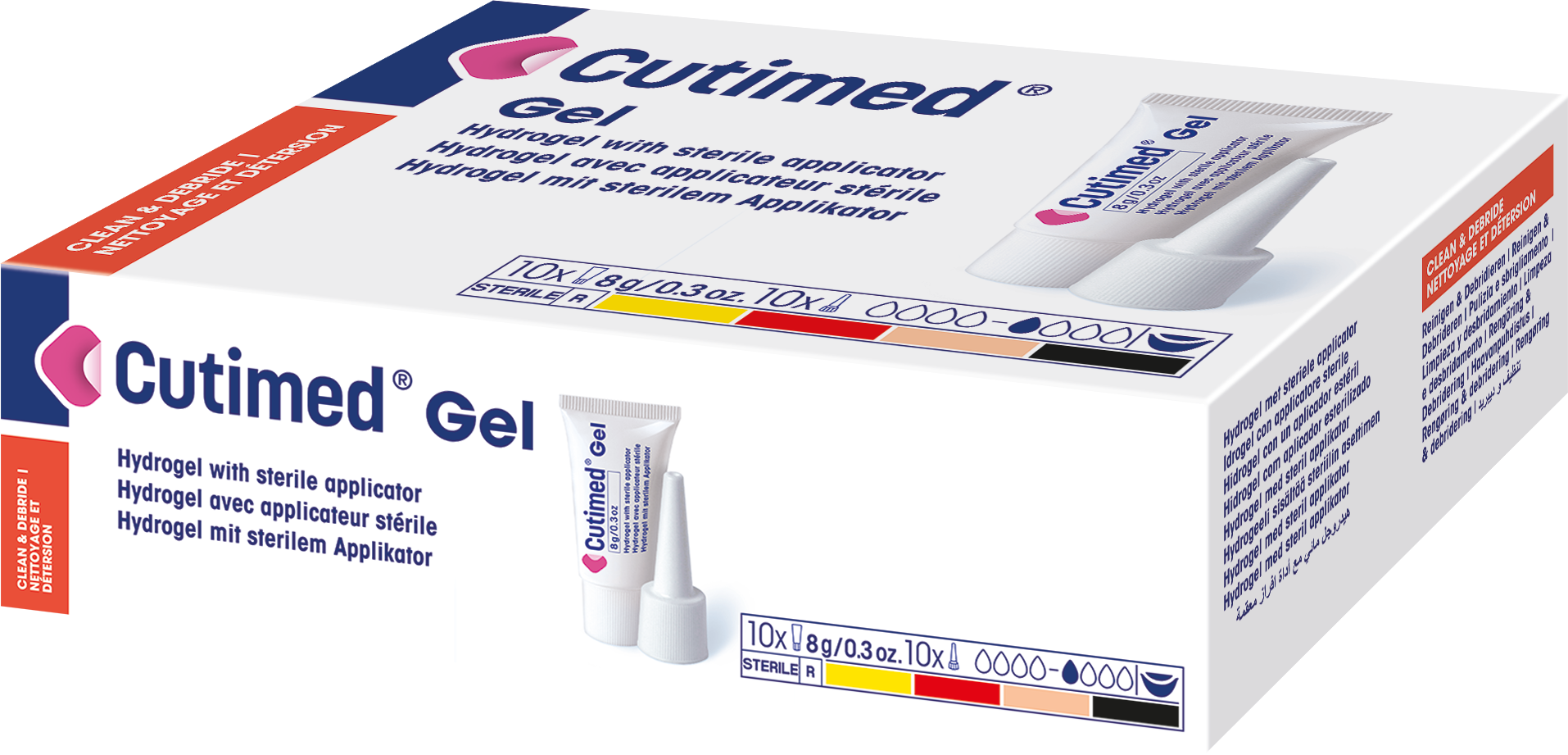
Cutimed® Gel
is a hydrogel with a sterile applicator.
Cutimed® Sorbact® Dressing Pad
is a sterile, bacteria and fungi binding absorbent wound dressing.
Cutimed® Sorbact® Ribbon Gauze
is a sterile, bacteria and fungi binding wound dressing.
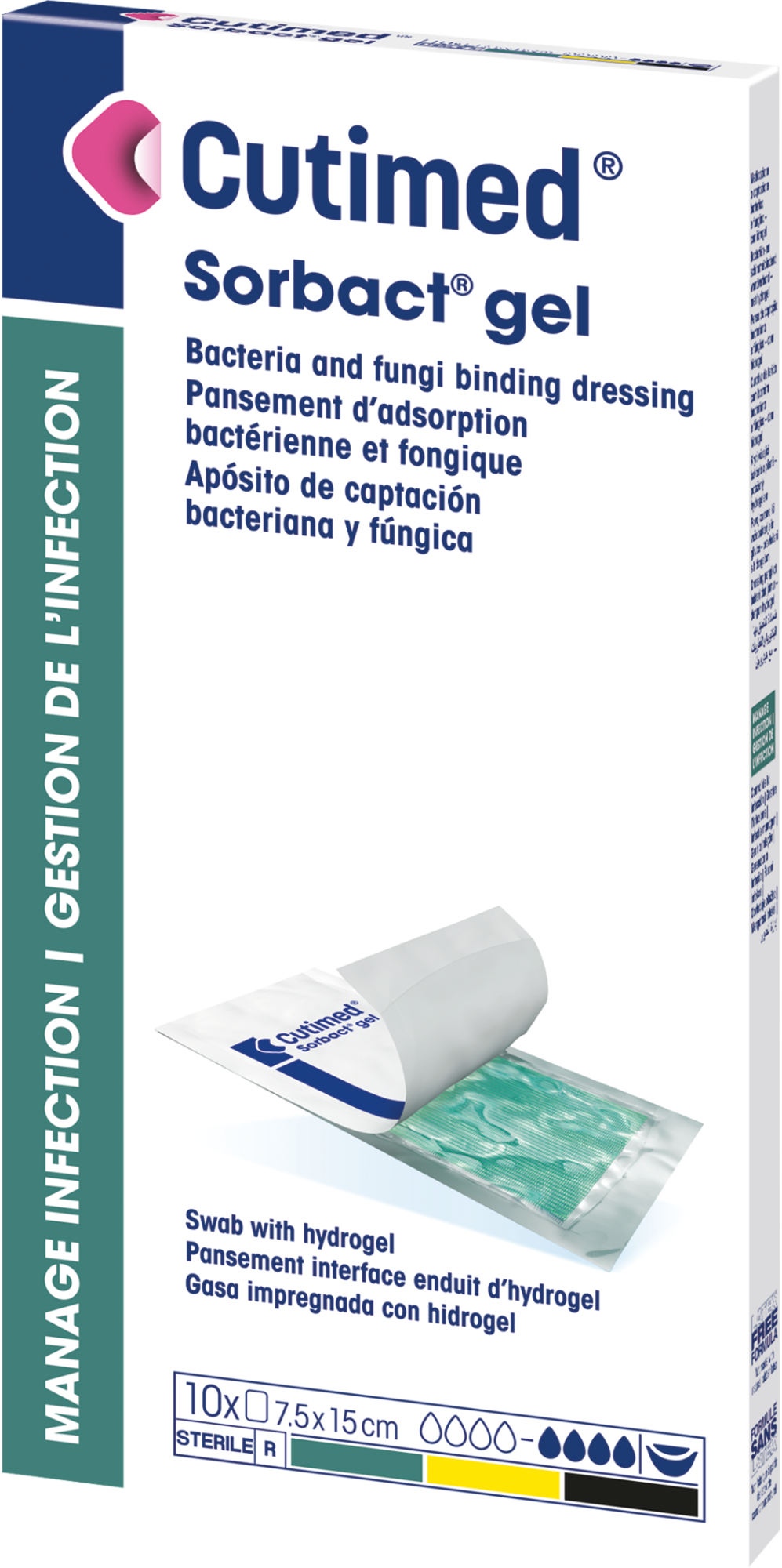
Cutimed® Sorbact® Gel
is a sterile, bacteria and fungi binding wound dressing with hydrogel.
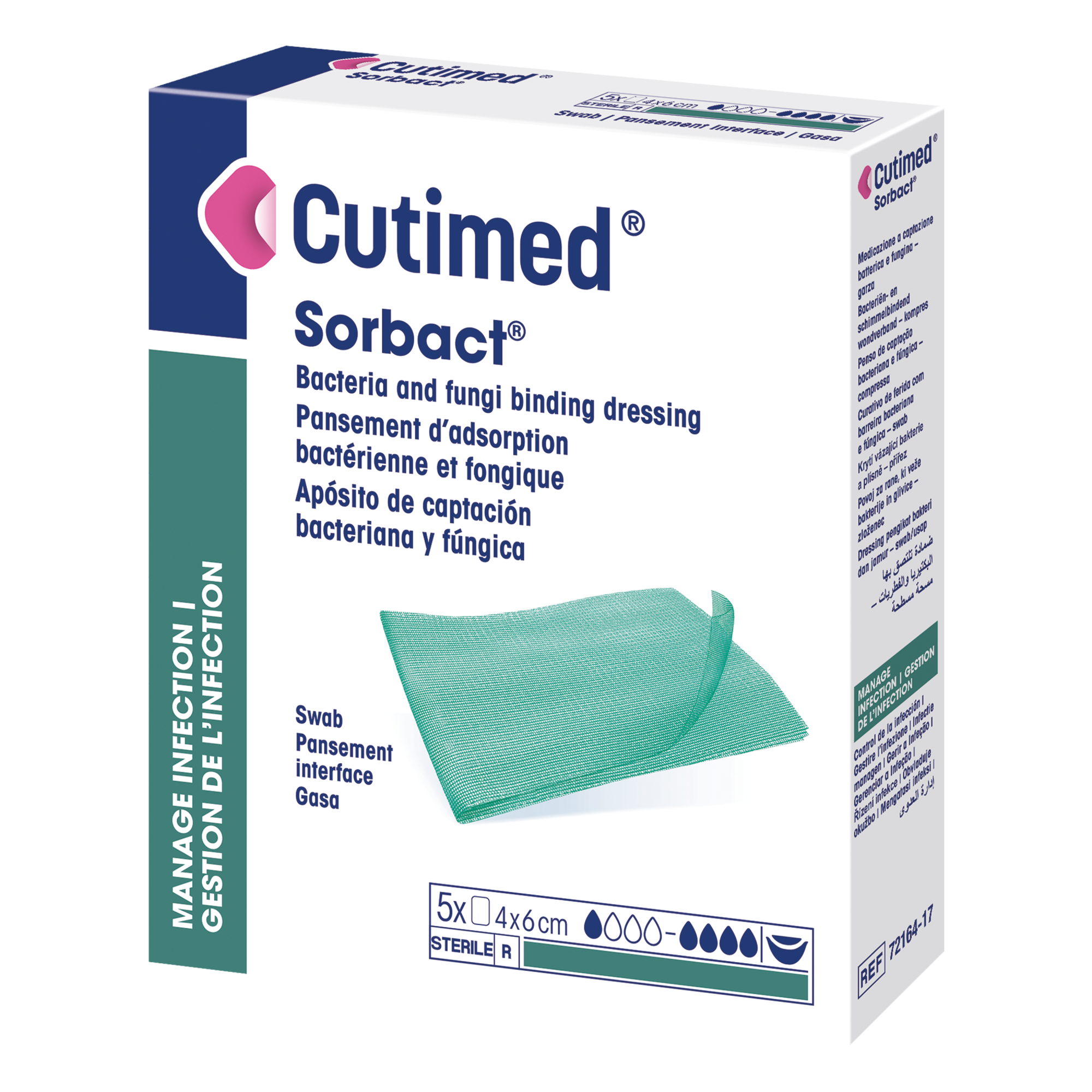
Cutimed® Sorbact® Swab
is a sterile, bacteria and fungi binding wound dressing.
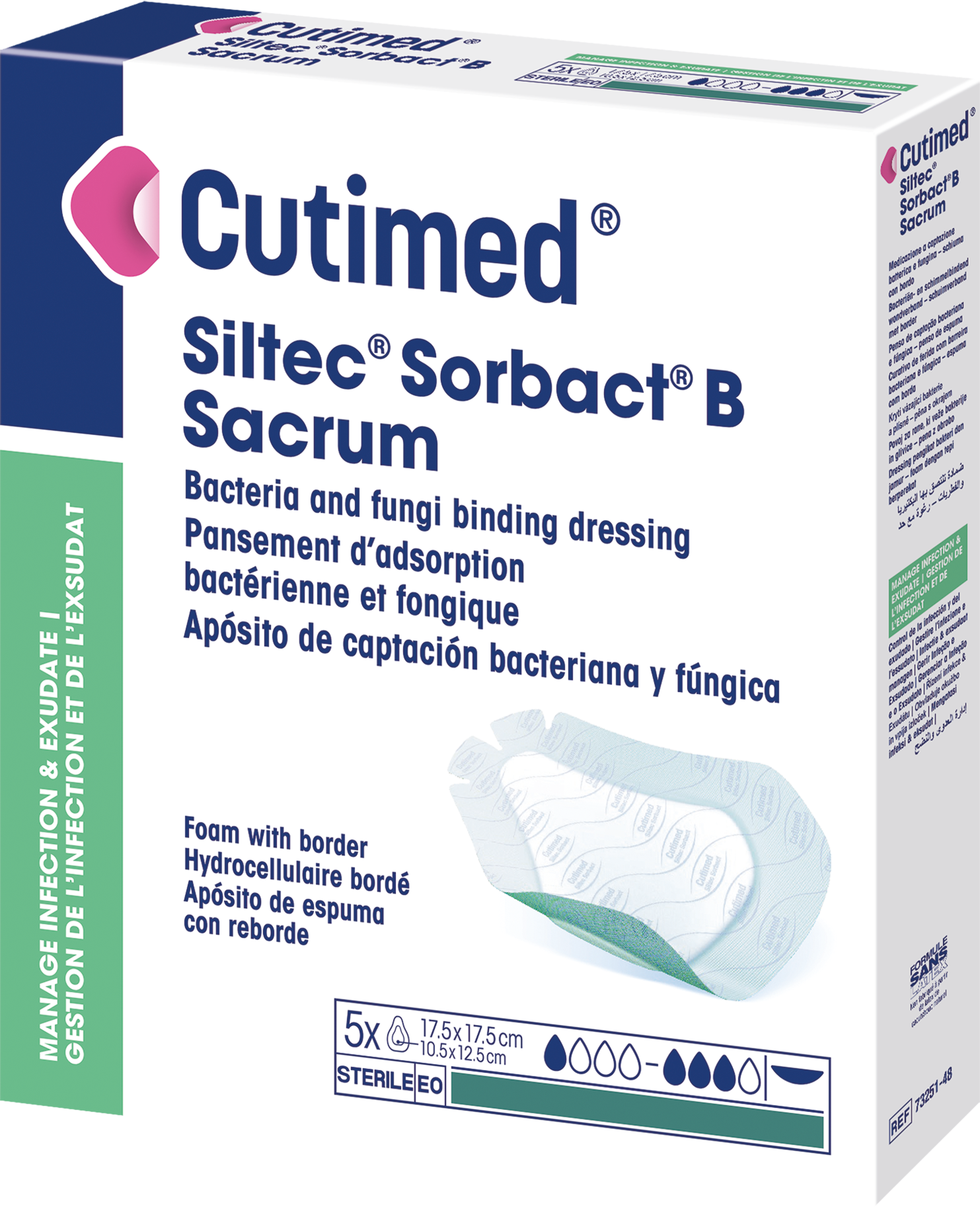
Cutimed® Siltec® Sorbact® B Sacrum
is a sterile, bacteria and fungi binding wound dressing with foam and a silicone adhesive border.
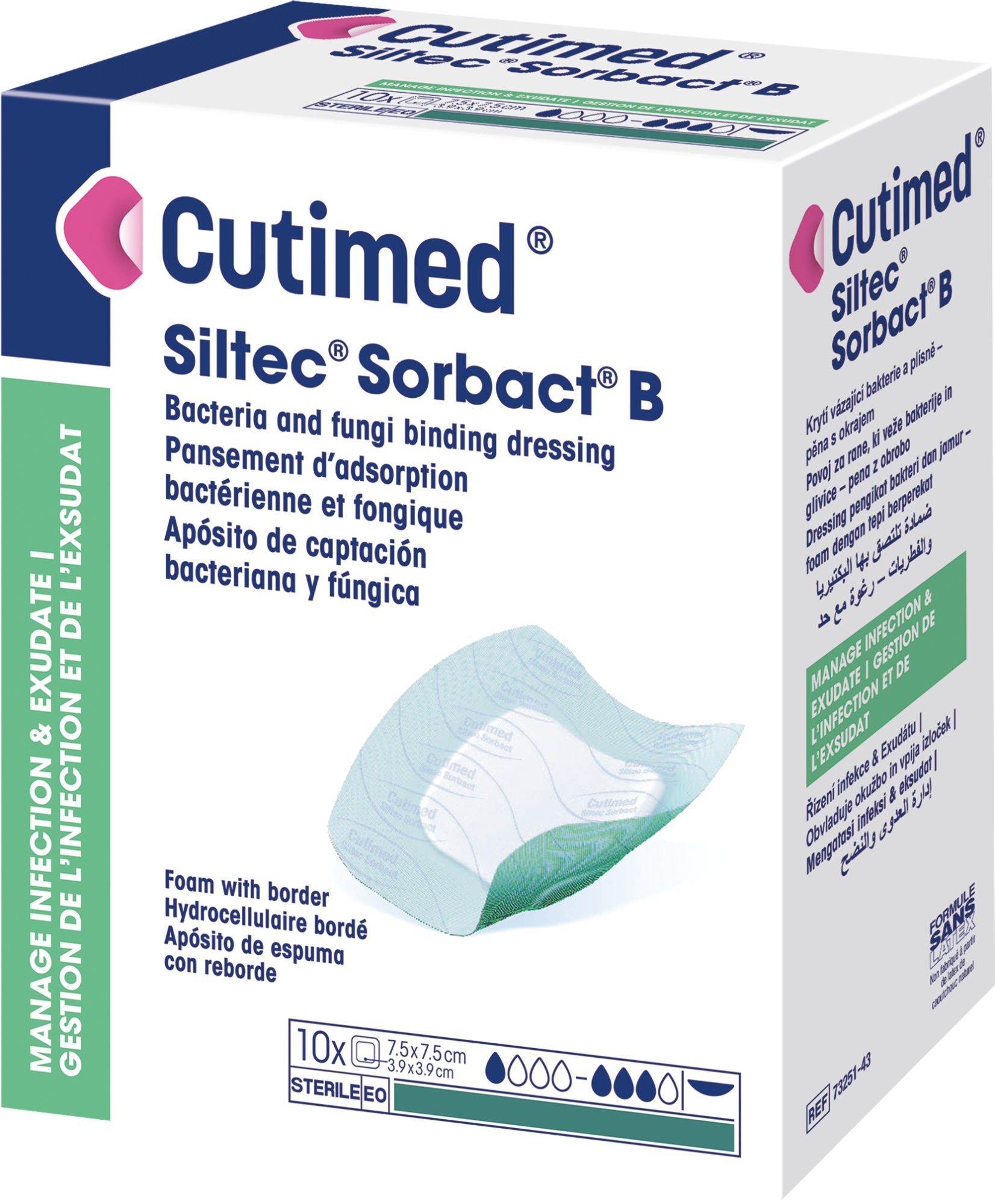
Cutimed® Siltec® Sorbact® B
is a sterile, bacteria and fungi binding wound dressing with foam and a silicone adhesive border.
Cutimed® Sorbact® Hydroactive
is a sterile, bacteria and fungi binding wound dressing with an absorbent hydropolymer matrix.
Cutimed® Sorbact® Hydroactive B
is a sterile, bacteria and fungi binding wound dressing with an absorbent hydropolymer matrix and an adhesive border.
Cutimed® Sorbion® Sorbact®
is a sterile, bacteria and fungi binding superabsorbent wound dressing.
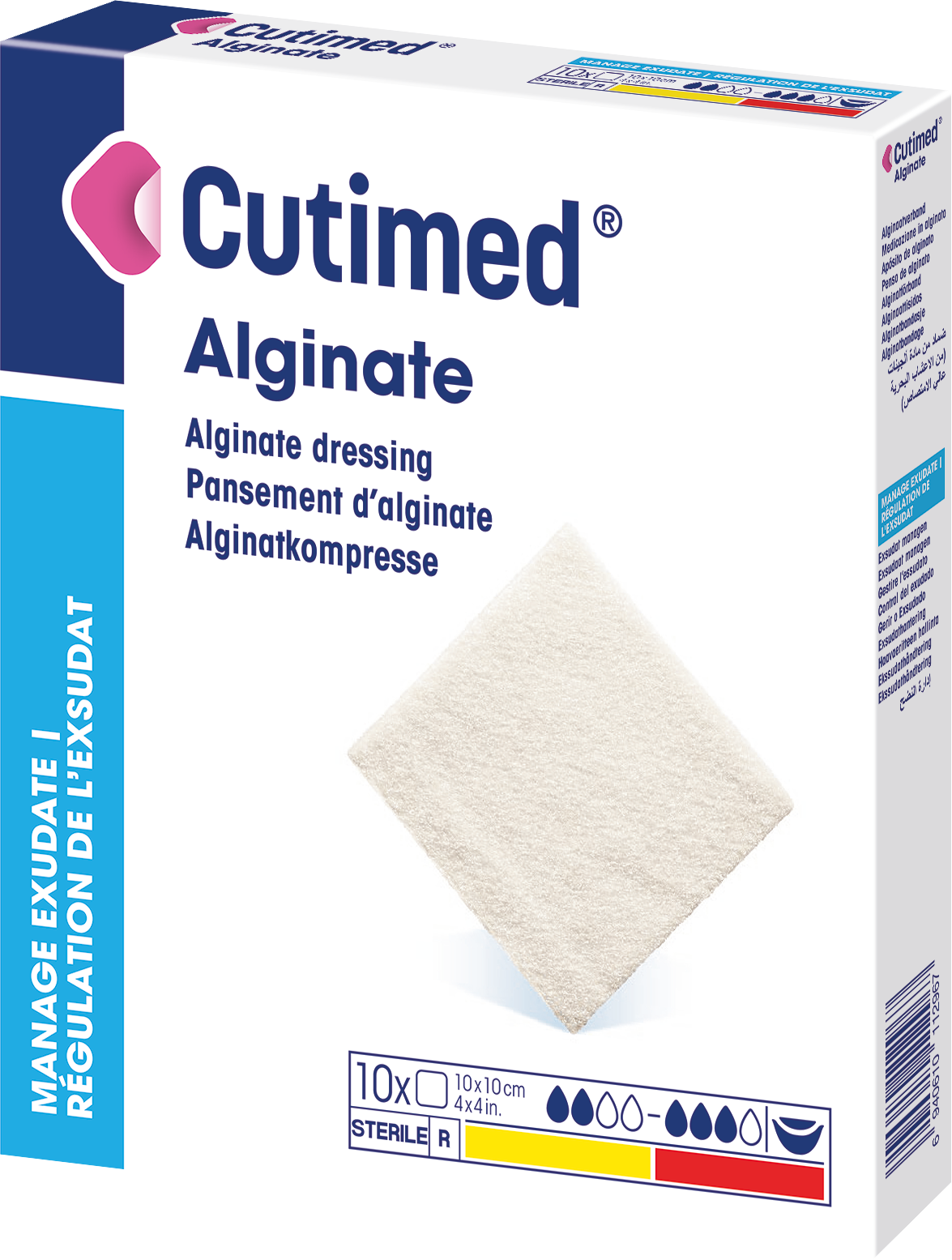
Cutimed® Alginate
is a sterile, non-medicated alginate dressing, consisting of mainly calcium alginate fiber.
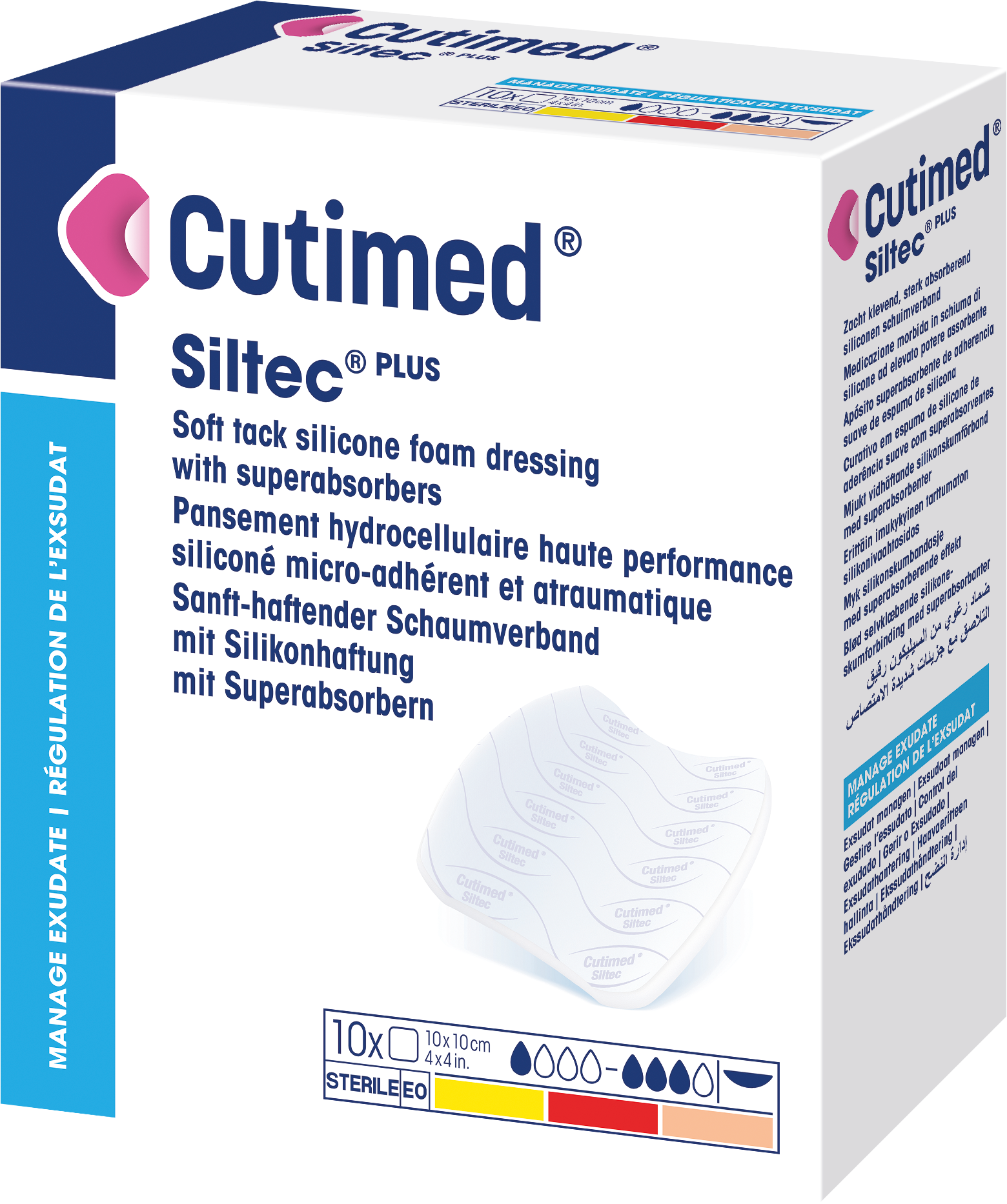
Cutimed® Siltec® PLUS
is a silicone coated, sterile, absorbent polyurethane foam dressing for atraumatic dressing changes that contains superabsorbent stripes which absorb and lock wound exudate.
Cutimed® Siltec®
is a silicone coated, sterile, absorbent polyurethane foam dressing with superabsorbent stripes to additionally absorb and lock wound exudate.
Cutimed® Gelling Fiber
is a soft, conformable CMC dressing (sodium carboxymethylcellulose) with enhanced fibers for strengthening.
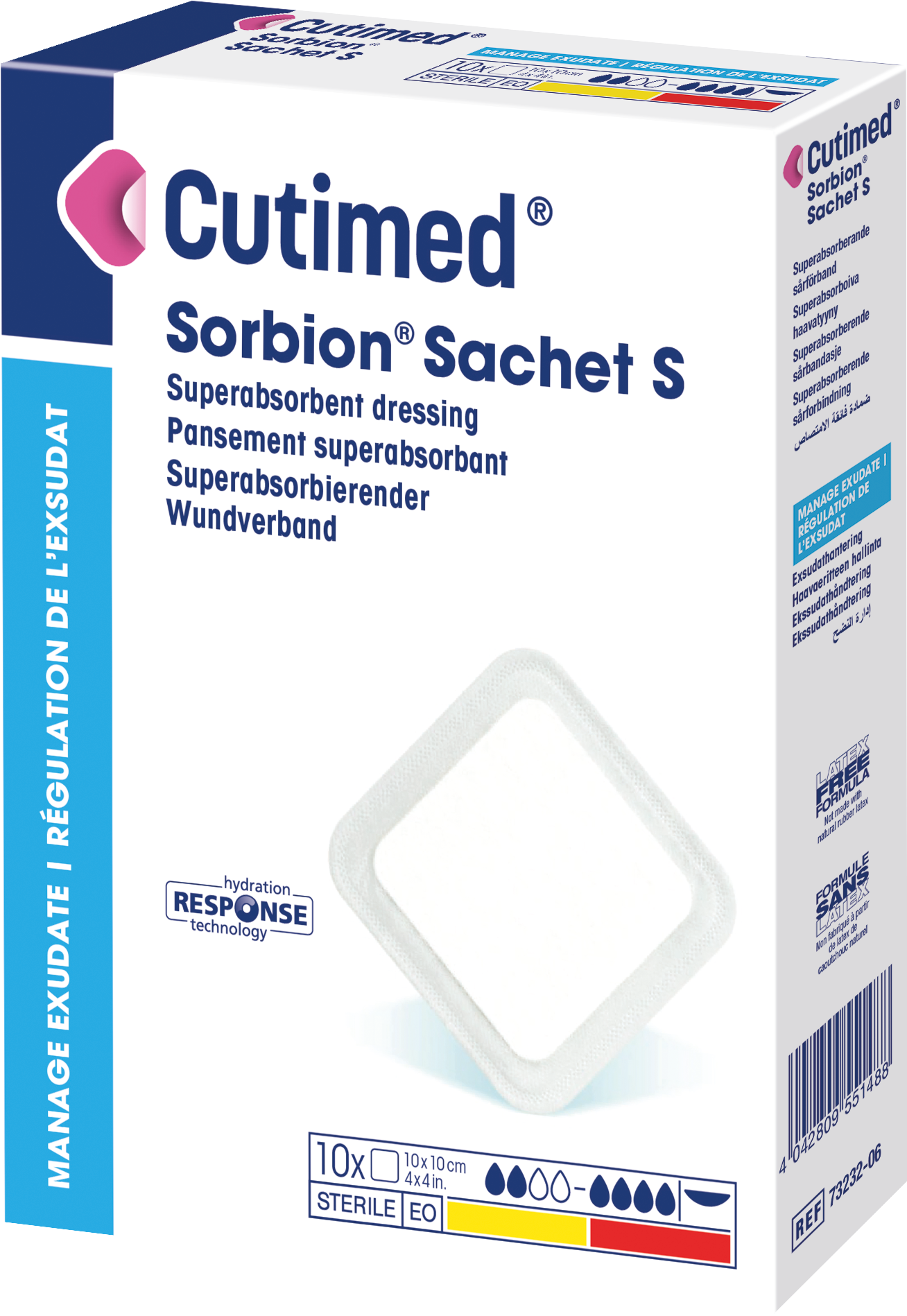
Cutimed® Sorbion® Sachet S
is a sterile, hydroactive, gel-forming, superabsorbent wound dressing.
Cutimed® Sorbion® Sachet XL
is a sterile, hydroactive, gel-forming, superabsorbent wound dressing.
Cutimed® Sorbion® Sachet Multi Star
is a sterile, hydroactive, gel-forming, superabsorbent wound dressing.
Cutimed® Sorbion® Sachet Extra
Is a sterile, hydroactive, gel-forming superabsorbent wound dressing.
Cutimed® Sorbion® Sachet S Drainage
is a sterile, hydroactive, gel-forming superabsorbent wound dressing.
Cutimed® PROTECT Spray
is a liquid skin protectant spray. The barrier film protects skin from irritation due to adhesive products or bodily waste and fluids.
Cutimed® PROTECT Foam Applicator
is a liquid skin protectant with applicator that forms a barrier film.
Cutimed® PROTECT Cream
is a barrier cream that protects skin from the damage and irritation.
Cutimed® Cuticell® Contact
is a primary wound contact layer consisting of an elastic and transparent polyurethane film coated with soft silicone.
Cutimed® Cuticell® Classic
is a sterile, non-medicated, paraffin gauze dressing with open weave.
Cutimed® Siltec® Sacrum
is a silicone coated, sterile, absorbent polyurethane foam dressing for atraumatic dressing changes that contains super-absorbent stripes which absorb and lock wound exudate.
Cutimed® Siltec® B
is a silicone coated, sterile, absorbent polyurethane foam dressing for atraumatic dressing changes that contains super-absorbent stripes which absorb and lock wound exudate.
Cutimed® Sorbion® Sana Gentle
is an atraumatic, sterile, hydroactive, gel-forming, superabsorbent wound dressing.
Cutimed® Siltec® L
is a silicone coated, sterile, absorbent polyurethane foam dressing for atraumatic dressing changes that contains superabsorbent stripes which absorb and lock wound exudate.
Cutimed® Siltec® PLUS Heel
is a silicone coated, sterile, absorbent polyurethane foam dressing for atraumatic dressing changes that contains superabsorbent stripes which absorb and lock wound exudate.